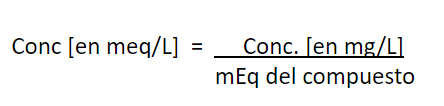What is water hardness?
Calcium and magnesium make up most of what is known as water hardness.
Technically, other multivalent cations (of valence greater than +1) are considered part of the hardness. However, its concentration is usually negligible in relation to that of calcium and magnesium.
The hardness of a water can be determined by titration, which is practical, inexpensive and simple. Inexpensive sets are sold to carry out this analysis.
Calcium hardness, magnesium hardness and total hardness are expressed in units of mEq/L or mg as CaCO3/L .
The terms
hardness
y
hard water
are due to the way multivalent cations, such as calcium and magnesium, attract and bind to the skin’s natural oils, leaving the skin feeling stiff.
In addition to the above, water hardness:
- Leaves hair stiff.
- It causes a characteristic taste (not pleasant for most people) in drinking water.
- It forms precipitation that encrusts or clogs water pipes and equipment such as heaters, irons and appliances. This precipitation increases as the water warms up.
- The positive charges of the hardness cause it to bind to phosphates, which are strongly anionic. Since the action of most detergents depends on phosphates or other anionic surfactants, hardness decreases their effectiveness.
- Hardness is also a problem for reverse osmosis equipment, due to its insolubility when the concentrations of bicarbonates, carbonates, sulfates, fluorides or silicates are appreciable.

Figure 1. Approach to pipelines affected by mineral scaling due to high water hardness concentration.
Effects of hard water
Hard water is rich in dissolved minerals, especially calcium and magnesium. You may have already felt the effects of hard water at some time in your life, I can feel as if there is a layer of residue left on your hands or dry skin and hair. Soap in hard water reacts with calcium (which has a relatively high calcium content in hard water) to form “foam”. When hard water is used, additional soap or detergent is needed to clean everything, be it hands, hair or clothes.
Also when washing dishes and glasses, have you noticed white spots or stains on your faucets or showerheads, these are from hard water deposits, which are not dangerous, but are unsightly or can clog pipes. Industrial water users have serious problems when their equipment or pipelines are heavily fouled.
When hard water is heated, the negative effect is multiplied and strengthened, as in domestic or industrial water heaters, a calcium carbonate deposit can form more quickly. It can shorten the life of industrial equipment and boilers, increase the cost of water heating, reduce the efficiency of electric water heaters and cause blockages in pipes. And yes, mineral buildup will also occur in restaurant coffee pots, which is why some people sometimes put vinegar (acid) in their coffee pots.
Conversion of units, from mg/L to mg
CaCO3/L

Example:
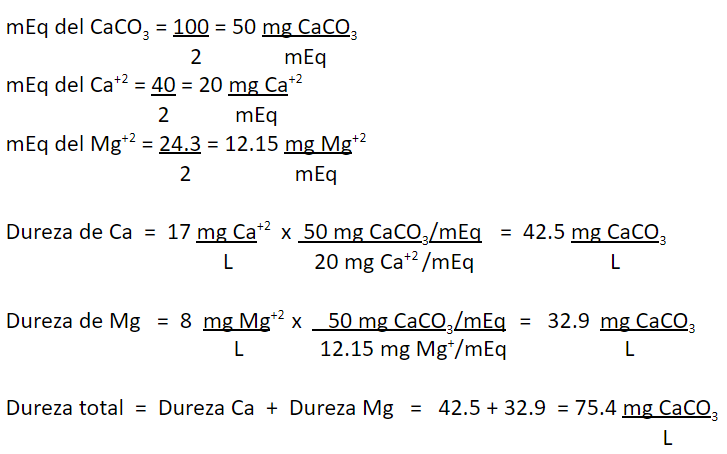
The concentration of Ca+2 concentration in certain water is 17 mg/L and Mg+2 is 8 mg/L. Express both concentrations in mg CaCO3/L and report the total hardness of this water in mg CaCO3/L.
Calculations:
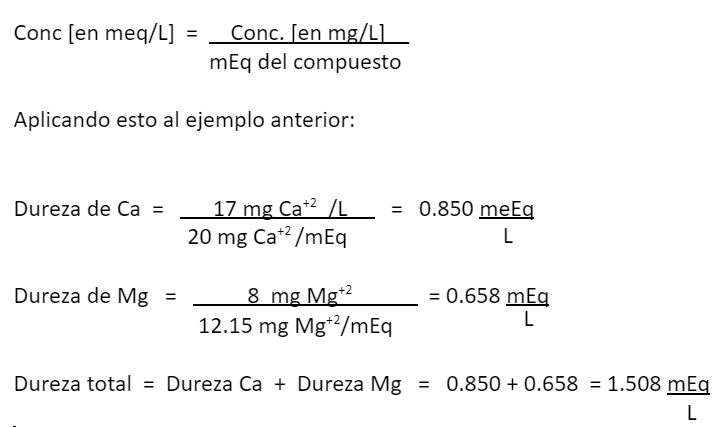
Conversion of units, from mg/L to mg CaCO
Applying this to the previous example:
How to measure water hardness
Hardness is composed of compounds of calcium, magnesium and other minerals on a smaller scale. A general guideline for water classification is that calcium carbonate is classified as soft;
Concentration as CaCO3
- 0 to 60 mg/L soft water
- 61 to 120 mg/L, moderately hard
- 121 to 180 mg/l, hard water
- above 180mg/L very hard water.
It is best to use some instruments or reagent kits to measure hardness by colorimetry.
Water hardness is the total concentration of calcium and magnesium ions in a water sample and is expressed as the concentration of calcium carbonate.
Temporary hardness is the part of the total hardness that is removed by boiling. Although not accepted as a standard method, the use of an ion selective electrode allows rapid measurement of water hardness and can be used to determine changes in hardness. Direct voltammetry methods are not recommended for ion selective electrodes, but indirect voltammetry methods, including titration with ethylene glycol acid, are recommended. The ion selective electrode used is a liquid ion exchange electrode that reacts with divalent magnesium and calcium ions.
Related articles: https://carbotecnia.info/aprendizaje/uncategorized/agua-dura-o-agua-blanda-diferencia-y-riesgo-a-la-salud/
-
RoQuest 4000 Coagulante líquido para materia orgánica Avista
Cotizar -
RoQuest 3000 Coagulante líquido orgánico de Avista
Cotizar -
Vitec 7400 de Avista para control de Sílice y Sulfatos
Cotizar -
RoClean L403 limpiador de incrustaciones por carbonato de calcio y metales de Avista
Cotizar -
RoClean P111 limpiador de membranas por bioincrustaciones
Cotizar -
RoClean P303 Limpiador de incrustaciones por carbonato de calcio y metales
Cotizar -
RoClean L211 limpieza de membranas con ensuciamiento de materia orgánica
Cotizar -
RoClean P112 Limpiador de membrana para Sílice SiO2
Cotizar