
pH of the water.
PH is a measure of the concentration of hydrogen ions (H+) in an aqueous solution. The term comes from the Latin pondus hydrogenii (“Amount of hydrogen”). We will not only focus on the pH of the water, but we will talk about the pH in general.
Later it is shown that the concentration of hydrogen ions has a direct relationship with the concentration of hydroxide ions (OH–), so the pH is also a measure of the concentration of the latter.
When an electrolyte dissolves in water, it generates a cation and an anion.
Water also dissociates and does so to a lesser or greater degree, depending on the electrolytes dissolved in it.
The equilibrium reaction representing the dissociation of water was originally believed to be:
H2O ???? H+ + OH–
H+ is the hydrogen ion, and OH– is the hydroxide ion. Today we know that the reaction is:
2 h2O ???? H3O+ + OH–
H3O+ is the hydronium ion, and OH– is the hydroxide ion. For practical purposes, even today, when we refer to the hydrogen ion, it is actually the hydronium ion. In accordance with this custom, from this point on, we will call what the hydronium ion really is the hydrogen ion.
Most dissociation reactions reach chemical equilibrium. When this is reached, the product of the concentration of the ions formed (in terms of molar concentration) is called the “reaction equilibrium constant”. In the case of water:
Kw = [H+] [OH– ]
Kw is the equilibrium constant of the reaction. For totally pure water and at 25 ° C, the molar concentration of hydrogen ions (in gmol / L) is:
[H+] = 1 x 10-7
And the molar concentration of hydroxide ions is:
[OH– ] = 1 x 10-7
Therefore:
Kw = [H+] [OH–] = 1 x 10-14 (1)
This relationship is true even if the water in question contains hydrogen ions or hydroxide ions from dissolved electrolytes that have been added to it.
Therefore, if the concentration of the hydrogen ion decreases, the concentration of the hydroxide ion increases by such a proportion that the product of both concentrations remains 1×10-14 at 25 ° C.
Since the numerical values of the concentrations of hydrogen ions or hydroxide ions in a solution are usually very small numbers, the chemist Søren Sørensen proposed pH as a unit of measurement for the concentration of hydrogen ions in aqueous solutions:
pH = – log [H+]
Water behaves in such a way that the range of values that the pH can have is from 0 to 14.
To measure the concentration of the hydroxide ion, pOH can be defined as:
pOH = -log [OH– ]
It is valid to obtain the negative value of the logarithm of each addend that makes up the equation (1):
-log Kw = -log [H+] – log [OH–] = -log (1 x 10-14)
-log Kw = -log [H+] – log [OH–] = 14
From the definition of pH and pOH, it can be inferred that:
pKw = pH + pOH = 14
There are equipment capable of measuring pH, called potentiometers. Considering the definition of pH, by increasing the concentration of hydrogen ions in the water, the pH decreases and by increasing the concentration of hydroxide ions, the pH increases.
It can also be seen that, if the pH decreased by one unit, the hydrogen ion concentration increased 10 times. In water with pH 5, the hydrogen ion concentration is 100 times higher than in water with pH 7.
The same applies to the opposite side: in a water with a pH of 13, the concentration of hydroxide ions is 100 times higher than in a water with a pH of 11.
As mentioned earlier, Kw it depends on the temperature. If the pH of an aqueous solution is measured at 25 ° C and another measurement is made to this same water at 50 ° C, the pH value will be different. The following table shows values of pKw values as a function of temperature.
Changes of the constant pKw depending on the temperature
|
Temperature (° C)
|
pKw = – log [Kw]
|
|
0
|
14.93
|
|
5
|
14.73
|
|
10
|
14.53
|
|
15
|
14.35
|
|
20
|
14.17
|
|
25
|
14.00
|
|
30
|
13.83
|
|
50
|
13.26
|
Examples of pH application
- With a volumetric pipette, we add 10 ml of sulfuric acid (H2SO4) to 98% in a 1-liter volumetric flask. We make up with distilled and degassed water. The laboratory temperature is 20 ° C. What pH will the solution have?
Answer:
H density2SO4 to 98% at 20 ° C: 1.84 g / cm3.
Mass of H2SO4 to added 98% = 10 cm3 x 1.84 g / cm3 = 18.4 g.
When percentages in liquids or solids are reported, if no clarification is made, it is referred to as % by weight. Therefore, the mass of pure sulfuric acid added to the flask is:
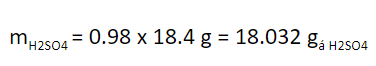
We convert to mole, dividing by the molar mass of H2SO4 (98 g / mol):
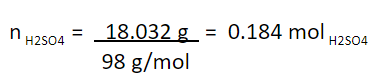
Molar concentration of the solution obtained: 0.184 mol H2SO4/ L.
Strong acids, such as sulfuric, completely dissociate in water. Therefore, all the hydrogen that is part of this acid will remain as an ion. From this, the hydrogen concentration in the solution will be:
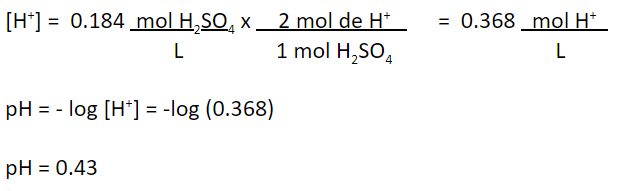
2. You have water with a pH of 13.7. It is known that it was obtained by mixing a 50% liquid soda (NaOH) solution with distilled and degassed water. The temperature is 25 ° C. What is the concentration of soda in the solution, in terms of mg NaOH / L?
Answer:
pH = 13.7
At 25 ° C,
pH + pOH = 14.0
pOH = 14 – pH = 14 – 13.7
pOH = 0.3
pOH = -log [OH–]
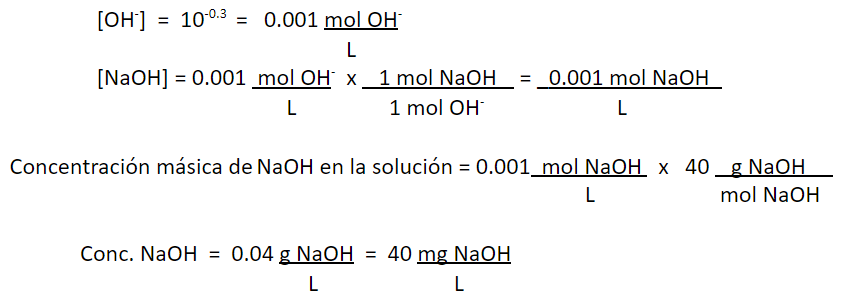

Source: https://es.wikipedia.org/wiki/PH
Comparte:
Necesitas más información, escríbenos.
Algunos productos que te pueden interesar
-
RoClean L211 cleaning of membranes with organic matter fouling
Add to quote -
RoQuest 3000 Organic Liquid Coagulant from Avista
Add to quote -
Titan Antifouling for Reverse Osmosis Membranes
Select options -
RoClean P112 Membrane Cleaner for Silica SiO2
Add to quote -
RoClean P303 Calcium Carbonate & Metal Scale Cleaner
Add to quote -
RoClean P111 Biofouling RO Membrane Cleaner
Add to quote -
RoClean L403 Calcium Carbonate and Metal Scale Cleaner by Avista
Add to quote -
RoQuest 4000 Liquid Coagulant for Organic Matter Avista
Add to quote























