What is iron?
Iron is a metallic chemical element, white, moldable and ductile, vital for animal and plant life, and the fourth most abundant element in the Earth’s crust. Its chemical symbol is Fe, has an atomic weight of 55.85 gmol / g. This metal is found in minerals such as hematite, magnetite, limonite, pyrite, etc.
It is considered a transition metal and is the fourth most abundant element in the earth’s crust, representing 5%, and among metals only aluminum is more abundant. In terms of planetary mass, iron is the most abundant metal because the planet concentrates in its core the greatest mass of native iron, equivalent to 70%.
The earth’s core consists mainly of iron and nickel in metallic form, generating a magnetic field as it moves.
Characteristics of iron
It is a malleable metal, has a gray to silver color and magnetic properties. It is a very hard and dense metal.
It can be found in nature because it is part of many minerals and is rarely found free. To obtain iron in its elemental state, the oxides must be reduced with carbon and then undergo a refining process to remove the impurities present.
Applications
Iron is considered the most widely used hard metal, accounting for 95% by weight of the world’s metal production. Pure iron does not have many applications, with the exception of using its magnetic potential.
Its main application is to form steel products, using this metal as a matrix element to house other alloying elements, both metallic and non-metallic. An iron alloy is considered to be steel if it contains less than 2.1% carbon; if the percentage is considered higher, it is called cast iron.
Difference between iron and iron
The truth is that fierro is another way of referring to iron, this is a type of metal.
The recommended form in the general language is hierro, since fierro is a variant of Latin American Spanish, in fact, it still retains similarity to the word that comes from the Latin “ferrum”.
Therefore, fierro is a way of calling iron in the American continent and is considered a colloquial and even cultured term.
Iron in Water.
Its presence in water is due to corrosion in pipes and storage tanks made of carbon steel containing a percentage of this element. In deep wells, when there is a high concentration of iron, it is due to an excess of iron minerals (such as hematite), which has contact with the water.
The reduction reaction of iron carbonate in well water is as follows:
FeCO3 +CO2 +H2O-> Fe+2 + 2HCO3-
This is an example of how this element dissolves in water in contact withCO2. This reaction is affected by pH, consequently, high pH increases chlorine precipitation and low pH increases the concentration of dissolved iron.
Health Effects
The concentrations of this metal found in water are generally do not present a health hazard. However, it can change the taste of the water, generate reddish-brown stains on clothes, as well as cause accumulation problems in pipes, pressurized tanks and even water softeners.
Bacteria.
Some types of bacteria obtain their energy by reacting with soluble forms of iron and manganese. These organisms are generally found in waters that have high levels of soluble iron. The reaction exchanges this soluble metal into a less soluble form of the metal, resulting in precipitation and an accumulation of black gelatinous material (slime). Masses of slime from this element can clog plumbing and water treatment equipment, in addition to breaking off in clumps that become iron stains in laundries. Bacterial reactions with iron do not cause any additional precipitation compared to exposure to an oxidant. However, precipitation caused by bacteria occurs faster and therefore increases the dyeing of fabrics quickly.
How does iron enter the water?
Being a common element on the surface of the earth. As water seeps into the ground it causes the rocks to dissolve and this mineral reaches the groundwater. Another way is that metal pipes often corrode and cause iron to reach the residential water supply.
How do you know if your water contains iron or iron?
One way to tell if your water contains iron or iron is by the taste of the water, another way to identify iron in water is that there are reddish-brownish particles that may be visible when the water comes out of the tap. Iron particles may come from corroded pipes or from the water supply.
The particles form because oxygen in the plumbing system may be oxidizing and precipitating the iron. The reddish-brown color of the water due to iron is caused by bacteria and can stain clothing and other items.
How to treat water to remove iron and manganese?
The following processes can be used to retain dissolved manganese in water:
– Iron-manganese catalysts with manganese dioxide:
This is the most efficient method used today and works through a process of oxidation-catalysis and filtration. The iron is oxidized to a soluble state with the help of an oxidant, such as chlorine or oxygen, and then on contact with a granular catalyst medium such as Z-ox, Greensand, Katalox it is bound by a catalyst and goes from being dissolved to becoming a solid, the same catalyst medium bed retains and is removed with a backwash. The surface of the catalyst has a porous structure that retains iron ions and other contaminants, allowing the treated water to flow through it. The effectiveness of these catalysts depends on several factors, such as iron concentration in the water, pressure, water flow and quality of the granular media.
– Phosphate treatment:
It is used against low levels of iron and manganese in the water and what is done is to inject phosphate compounds into the water system. Phosphate helps prevent minerals from oxidizing and helps keep them in solution.
The way to introduce the phosphate elements into the water is when the iron is dissolved in order to keep the water clear and prevent staining. Phosphate treatment is relatively inexpensive and may not be very effective.
– Ion exchange water softener:
This method is effective for low iron or iron levels, this type of water is commonly treated with an ion exchange water softener. Not all water softeners can remove iron from the water, in case of treating water with excessive levels of iron they can clog the softener.
– Oxidizing filter:
The filter is basically natural manganese greensand or synthetic zeolite that absorb dissolved iron.
– Raising the pH and particle filtration:
The simplest solution against pipe corrosion is to raise the pH of the water and use a sediment filter.
– Shock treatment:
It is the most common method of killing bacteria and chlorine is the most commonly used chemical for this process. This treatment is used repeatedly as it is impossible to kill all the iron bacteria in a system, so they are likely to grow back.
– Multi-step treatment:
This treatment works with high levels of iron in dissolved or solid form. The first step is to add chlorine to the water to oxidize the dissolved iron and kill bacteria. Another step is to filter the water through a mechanical device that removes particles. It can then be filtered with activated carbon to remove chlorine and the last step is to soften and remove dissolved minerals.
– Aeration:
This method is recommended for water with high iron concentration in order to reduce reagent costs. The equipment normally used in this type of method is an aerator, a holding tank and filters. Oxygen from the atmosphere reacts with the iron in the raw water to produce relatively insoluble oxides of these elements. The reaction rate depends on the pH of the solution, being faster at higher pH values.
– Oxidation:
It consists of a chemical dosing system and filters. A holding tank and a system for adjusting the pH with sodium hydroxide, calcium hydroxide, hydrated lime or sodium carbonate may be required. Oxidizing agents such as chlorine gas or hypochlorite may be used.
– Filtration in conditioned media:
Filter media conditioned to remove iron have a regeneration, adsorption and filtration capacity that depends on the particle size distribution of their shape and surface manganese oxide precipitates. Potassium permanganate is used as an oxidizing agent, which has a high cost and must be very well controlled in its application due to its toxicity.
– Direct filtration with the application of chemicals:
The application of chemicals is required to agglomerate the oxidized particles and form flocs large enough to be filtered.
– Alternative technologies:
Softening is applied in order to remove existing hardness in the water where iron removal is a secondary effect. This method is not very efficient since precipitates are formed when coagulants are not added.
– Stabilization due to sequestration:
Sequestering agents are chemicals that help prevent metals from precipitating. Normally, both sodium silicate and polyphosphates are used to sequester iron. The sequestering agents do not remove iron, they only prevent it from precipitating and their application is recommended in small systems and concentrations of less than 0.5 mg/L.
– Biological methods:
Biological treatments always require specific raw water qualities and conditions and not all groundwater or surface water is economically feasible to treat.
Related articles:
Reference:
10 Water purification methods
If you need more information, please contact us.
Some products that may interest you
-
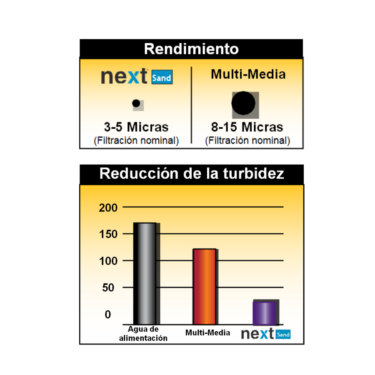
Turbidex Zeolite
Read more -
Filox – R for iron and manganese removal
Add to quote -
Garnet or Garnet deep bed filter media
Add to quote -
Anthracite – filter media for multimedia filters
Add to quote -
Zeolite for water filtration NextSand
Add to quote -
Silica Gravel Filter Media
Add to quote -
Silica Sand Filter Media
Add to quote