Description
NextSand® brand Zeolite for water filters
What is Zeolite?
Zeolites are porous minerals of volcanic origin, consisting of a scaffold of silicon and aluminum tetrahedra, arranged in an orderly fashion and joined by oxygen atoms, which are the skeleton of their crystalline structure. in the open spaces of the latter, whose pore diameter ranges from 3 to 10 angstroms, water molecules are introduced without participating in the cohesion of the network.
Natural absorbent due to its high degree of hydration. Excellent stability in its crystalline structure when dehydrated. Its density is low and it retains a large volume of voids when dehydrated.
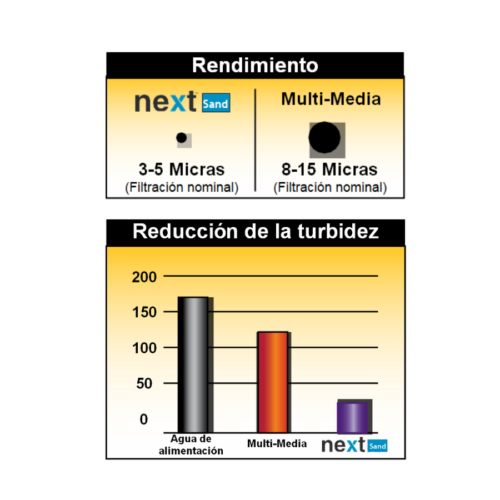
High capacity collector, retains particles up to 5 microns.
Origin and properties of zeolite
The term zeolite was originally coined in 1756 by the Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapid heating of the material, which was believed to be stilbite, produced large amounts of water vapor that had been adsorbed by the material. Based on this, he named the material zeolite, from the Greek ζέω (zéō), meaning “to boil” and λίθος (líthos), meaning “stone”.
Zeolites are a mineral with a relatively open three-dimensional crystal structure constructed from the elements aluminum, oxygen and silicon, with alkali or alkaline earth metals (such as sodium, potassium and magnesium) and water molecules trapped in the gaps between them.
The most interesting thing about zeolites is their open, cage-like structure and the way they can trap other molecules inside. This is how water molecules and alkali or alkaline earth metal ions (positively charged atoms with very few electrons, sometimes called cations) become part of zeolite crystals, although they do not necessarily remain there permanently. Zeolites can exchange other positively charged ions for the metal ions originally trapped within them (technically, this is known as cation exchange) and, as Cronstedt discovered over 250 years ago, they can gain or lose their water molecules very easily. Zeolites have regular sized openings, which allow small molecules to pass directly through, but trap larger ones; this is why they are sometimes referred to as molecular sieves.
Natural zeolite can be modified by a single or combined treatment, such as heating and chemical modification (acids, bases and inorganic salts). Chemical and thermal treatment of zeolite can cause cation migration and thus affect cation location and pore opening. “Pore engineering” is a popular term for methods used in zeolite modification in which some of its adsorptive properties are manipulated. The processes of ion exchange and adsorption in contact of the zeolite with the solution occur at the same time.
High temperature treatment, depending on the solid sample and the temperature used, can improve pore volume by removing water molecules and organic substances from the pore channels. Water present in zeolite cages and channels contributes 10 to 25% to the total mass of zeolites. To enable the efficient use of zeolites in water treatment, it is important to know the dehydration properties and structural stability of certain zeolitic materials.
What is zeolite used for?
Natural zeolite allows the filtration of drinking water in deep bed filters without the need to combine it with other filter media, since it meets the specifications of giving depth to the filter bed and retaining particles between 5 and 10 microns.
The cation exchange properties of zeolite neutralize certain elements. Uniform molecular channels are sorted in order upon dehydration.
High absorption capacity of gases and vapors. It has catalytic properties and ion exchange capacity.
Upon dehydration, the structure forms open three-dimensional networks and its compounds are arranged in channels of molecular order size, presenting an excess of negative charge as a consequence of the isomorphic substitution of Si4+ by AI3+, thus developing its gas and vapor absorption capacity and allowing ion exchange.
In this phenomenon, also known as cation exchange, ions are displaced by ammonia or heavy metal ions.
Zeolite NextSand EF11 for water filter.
Improved particle removal.
Improved water filtration performance results in higher water quality and cost savings.
Over the past 50 years, improvements in water filtration with granular media have been achieved by:
- The use of smaller mesh size sand to remove finer particles.
- The combination of sand with garnet or garnet and anthracite (i.e., “multimedia” beds), which has resulted in a lower nominal filtration range of 12-15µ for multimedia.
- Improved fluidic design of beds and vessels.
Further improvements in the performance of granular filter media can be achieved by modifying the particle characteristics in the feed water stream or by increasing the filter media surface structure and/or surface area to improve particle inertial impaction.
Filter media hardness and longevity
The hardness, i.e. the abrasion resistance of zeolite filter media is determined by the purity of the clinoptilolite mineral, with higher purity being a favorable characteristic. In addition, the binding (or cementing/bonding) properties of the mineral impurities in NextSand granules are important for abrasion resistance and water insolubility.
The rare high purity of nextSand mineral establishes the longevity of the filter bed. It is the most durable filter media (more than 5 years).
In addition, high purity NextSand media has the quality and purity required for reliable filtration.
Benefits of Natural Zeolite
- It reduces a little the acidity of the water.
- A smaller amount of product is required with respect to silica sand due to its porosity and density.
- It has a greater surface area and porosity.
- Produces greater clarity in filtered water.
- It is the most durable filter media (more than 5 years).
- It only requires a simple periodic backwash to maintain its efficiency and performance.
- It has a flow capacity 4 times higher than that of conventional filter media.
- Increases flow in equipment with multimedia, gravity and pressure systems compared to sand filters.
- Very few backwash cycles of the zeolite filter are required, resulting in significant energy savings.
- It has a higher retention capacity due to its larger surface area.
- Highly effective for filtering and purifying water.
- Economic
- Eliminates organic matter.
- Abrasion resistant
- Dehydrates sludge
- Requires 50% fewer backwash cycles than sand.
- Reduces the amount of backwash water due to fewer backwash cycles
- Classified as GRAS (generally regarded as safe) for humans and animals
- Non-toxic and environmentally friendly.
- It does not contain chemical additives or colorants.
Zeolite applications for filtration
- Deep bed zeolite filters for water purification.
- Filters for sediments larger than 5 microns.
- Industrial filtration for production lines.
- Tertiary wastewater treatment.
- Water filter for water purification plants, hotels, hospitals, restaurants, Etc.
Physical properties
- Compounding: High purity aluminum-silicate.
- Size: 4-1.4 mm (approx. 14×40 mesh).
- Color: Dark gray.
- Surface area: 25m2/gram.
- Surface absorption: Hydrophilic.
- Thermal stability: Stable up to 500° C.
- Uniformity coefficient: 1.7.
- Bed vacuum volume: 55%.
- Surface charge: Net negative.
- Bulk density: 55 lbs/ft3 (0.88 kg/L).
- Packaging: 1 ft3, Super sacks 1 m3.
Performance characteristics:
- Filtration (nominal) 3-5 microns
- Surface load 16-20 gpm/ft2 (Typical) – 12 gpm/ft2 (Optimized for silt, SDI and ultrafine particles)
Learn more about deep bed filtration in the following link: https://carbotecnia.info/aprendizaje/filtracion-de-agua-liquidos/filtros-de-lecho-profundo/
Source: https://www.filtrashop.com/10-metodos-de-purificacion-de-agua-mas-usados-y-efectivos/
Next Sand Material Safety Data Sheet:
https://www.carbotecnia.info/PDF/medfiltrantes/hoja-de-datos-de-seguridad-zeolita-next-sand.pdf