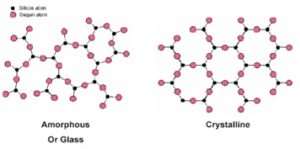
What is silica?
Silica is the conventional name for the chemical compound of silicon oxide, SiO2 in the solid state. Including both the crystalline solid state (they have defined and repetitive patterns in the distribution of their particles) and the amorphous state (they have no defined order).
There are different types of crystalline states for silica, the following figure shows the three main crystalline states found in nature: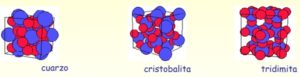
Each of these types contains different properties due to their distribution in space alone.
This material is widely used in water treatment as a granular medium in the deep bed as quartz sand (or silica sand) for the retention of suspended particles in colloidal form. since the solubility of quartz in water is too small (1.2 mg/l), so much so that it is considered to be insoluble.
Problem of silica in water.
Despite using this material as a filter media, silica in its other forms begins to present a problem in the water. as it can dissolve in water, or be suspended in particles of small diameter (d<1 micron).
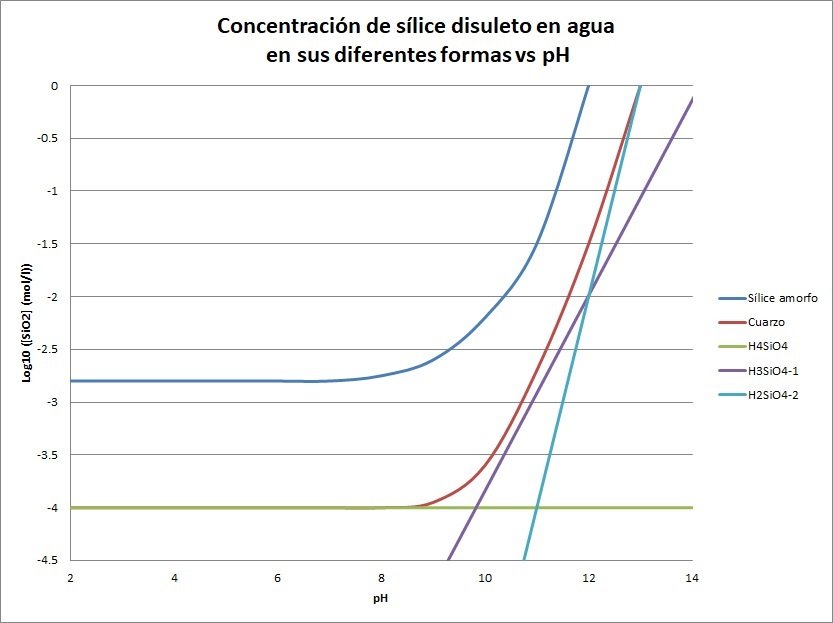
The amount of dissolved and colloidal silica depends strongly on the pH as a function of silicic acid (H4SiO4), which behaves as a weak acid that partially dissociates into different species.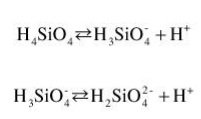
The following graph shows how an increase in pH results in more dissolved silica species in the water. (The graph is logarithmic with base 10, so the closer the value is to 0, the higher the concentration).
Table: Concentration of dissolved silica in water in its different forms vs pH.
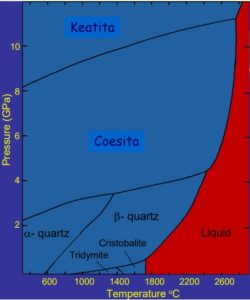
The incrustation and precipitation of silica in crystalline state depends on both pressure and temperature, so they tend to appear in reverse osmosis membranes working at high pressures.
The following graph shows the formation of the crystalline states of silica as a function of pressure.
What are the treatment methods for silica in water?
Reverse osmosis.
Removes salts, metals and minerals. Reverse osmosis separates ions with the help of charged particles, which means that dissolved ions that have a charge, such as salts, are more likely to be rejected by the membrane. A polymer thin film membrane rejects more than 96%, and a cellulose acetate membrane rejects about 85%. So it is a very good system for removing silica from water.
But also silica can significantly affect reverse osmosis membranes. The presence of silica in the water can lead to the formation of a silica (silicate) layer on the surface of the reverse osmosis membrane. This phenomenon is known as “fouling” and can reduce the efficiency and visa time of reverse osmosis membranes, increasing the pressure required for water treatment and decreasing the quality of the water produced.
The concentration of silica in water that can begin to be detrimental to a reverse osmosis membrane varies depending on the type of membrane and operating conditions. Generally, it is considered that silica concentrations of more than 20-30 mg/L can become problematic. However, this value may be lower under conditions of higher recoveries or higher temperature, as these conditions favor silica precipitation.
To manage high silica concentrations, measures such as water pretreatment can be taken to reduce the silica concentration, the use of specific antiscalants for silicaThe operation of reverse osmosis systems at lower recovery rates to minimize the concentration of silica in the retained solution. Silica antiscalants are generally effective in keeping silica in solution and preventing it from precipitating and sticking to membranes. They can significantly increase the threshold silica concentration at which fouling occurs, thereby reducing the frequency and severity of fouling.
It is important to note that these values and recommendations may vary according to the membrane manufacturer’s specifications and the specific conditions of each water treatment system. Therefore, it is always advisable to consult the technical documentation of the reverse osmosis system and, if necessary, and we can help you choose the best product for your reverse osmosis system.
Anionic resins.
Anionic resins can be designed to retain silica since the dissolved species are weak anions. These resins work through an ion exchange process. This method is especially useful in applications where a significant reduction in silica concentration is required.
The most common ion exchange resins for removal are strong base anionic resins. These resins are effective in capturing silica ions, especially in their silicic acid form (H4SiO4), which is the most common form of silica in aqueous solutions at neutral or slightly acidic pH.
There are several factors to consider when using ion exchange resins for silica removal:
Exchange capacity: The exchange capacity of the resin determines how much silica can be removed before the resin needs to be regenerated.
Regeneration: Resins must be regenerated periodically to maintain their effectiveness. Regeneration involves washing the resin with a solution that replaces the captured silica ions with ions that can be exchanged back into the water.
3. Water conditions: pH, temperature and the presence of other ions in the water can affect the efficiency of the resin. For example, a higher pH may decrease the efficiency of the resin in capturing silica.
4. Resin type: There are different types of anionic resins, and the appropriate selection depends on the specific characteristics of the water to be treated and the water quality objectives.
5. Cost and maintenance: The use of ion exchange resins involves initial installation costs and ongoing operation and maintenance costs, including resin regeneration.
Ion exchange resins are a viable option for silica reduction in water, but require careful selection and proper handling to be effective. In addition, it is important to consider the balance between the costs and benefits of its use compared to other water treatment technologies.
Related articles:
- Glossary of water treatment terms
- Inorganic contaminants
- Significance of Total Dissolved Solids in Water (TDS)
Share:
If you need more information, please contact us:
Source https://es.wikipedia.org/wiki/%C3%93xido_de_silicio(IV)