Water filtration
The first recorded attempts at water filtration date back to 2000 BC. The earliest Sanskrit writings described methods for purifying water. These methods ranged from using fibers or fabrics such as a very rustic sand filter, as well as boiling or placing hot metal instruments in the water before drinking.
Water filtration is a basic principle of using a medium (membrane, mesh, screen or other media) to physically filter or trap particles depending on their size. These filters are classified according to the pore size of a mesh or membrane, which is measured in microns; the smaller the number of microns (i.e., the smaller the pore size), the more it will retain sediment from the liquid being filtered.
Filtration is a solid-liquid separation process used in water treatment systems to reduce the concentration of suspended solids (particles) in the water. There are several types of filters and each of them has its application depending on the size and concentration of particles to be retained. In the
Figure 1
the different types of filtration and the typical particle size they retain can be seen.
What is filtration?
Filtering consists of separating solids that are suspended in a fluid. The fluid can be a liquid or a gas. Separation is achieved through a porous medium, such as:
- Perforated metal basket.
- Metallic sieve: it is a mesh. It is also called sieve or sieve.
- Bed formed by granular particles (sand, zeolite, anthracite, garnet…).
- Fabric woven from polymer yarns or natural fibers, such as cotton: used in bag filters or filter presses.
- Paper: as used in laboratories or filter presses.
- Cardboard: as used in filter presses.
- Discs.
- Cartridge of a porous material (yarn, pleated, polymer foam)
- Membranes (microfiltration, ultrafiltration, nanofiltration and reverse osmosis).
The porous medium retains the larger solids at the opening of its pores and allows the passage of smaller particles and liquid.
The chemical engineering career was created at the beginning of the 20th century with the aim of professionalizing the approach (study, design, operation) of industrial processes, and divided them in two:
- Those in which a chemical reaction occurred, he called unitary processes.
- Those in which only physical or physicochemical changes occurred were called unitary operations.
Difference between unitary operations and unitary process.
Unit operation |
Unit process |
Stages of the process where purely physical changes occur |
Process steps where a chemical reaction occurs |
Examples: |
Examples: |
Distillation |
Oxidation |
Evaporation |
Combustion |
Drying |
Pyrolysis |
Extraction |
Hydrogenation |
Filtration |
Polymerization |
Crystallization |
Fermentation |
Absorption |
Saponification |
Humidification |
Electrolysis |
Etc. |
Etc. |
The term unitary refers to the fact that the principles governing each of the processes or operations in question are the same, regardless of the industry in which they are applied.
For example, the principles governing fermentation are the same, regardless of whether it occurs to make alcohol from sugar cane, grapes or hydrolyzed agave. And the principles governing the distillation of petroleum are the same as those governing the distillation of grape wine, cider or agave fermented wine.
Among the unit operations, an important group is those applied to separate components of a mixture. They are so important that the chemical engineering curriculum devotes more time to them than to unit processes (in which a chemical reaction occurs).
When a manufacturing method has a chemical reaction as an essential step, it is often more challenging and costly to separate the final product, consisting of: the desired compound, unwanted by-products, unreacted reagents and inert compounds that were part of the reagents.
Over time, in the everyday language of engineering and industry, unit operations aimed at separation have been called separation or purification processes.
Perhaps it would be more accurate to call chemical engineering separation process engineering.
Among the unitary operations not intended for separation are those for: transporting fluids, transferring heat (for heating or cooling), stirring, mixing, grinding…
As far as we go: filtration is a unitary operation whose purpose is the separation of solids that are suspended in a fluid. And in colloquial engineering parlance, filtration is a separation process.
Among the separation processes applied by humans, filtration is by far the most widely used. Drying and adsorption on activated carbon are far behind. And far behind the latter is distillation, not to mention others.
What are membrane separation processes?
Since the origin of chemical engineering, the greatest technological innovation in the separation processes are those carried out by means of membranes. These began to be used massively in the last two decades of the 20th century. They are applied to the treatment of water and aqueous solutions . Four types of membranes are produced:
Membrane |
Approximate range of molecule, ion or particle diameters retained
(µ, microns) |
Microfiltration |
0.10 a 1.0 |
Ultrafiltration |
0.01 a 0.10 |
Nanofiltration |
0.001 a 0.01 |
Reverse osmosis (also called “hyperfiltration”) |
0.0001 a 0.001 |
Unit of length measurement |
Equivalent to: |
1 m |
1000 mm |
1 mm |
1000 µ or µm |
1 µ or µm |
1000 nm (nanometers) |
1 nm |
10 Å (angstroms) |
The micron (µ) or micrometer (µm) is one thousandth of a millimeter (mm).
Microfiltration and ultrafiltration membranes are porous. Therefore, they retain particles that are larger than their pore size.
Nanofiltration membranes are porous, but also allow particles (including molecules) to pass through by the dissolution-diffusion mechanism (also known as “translocation”). The latter consists of the particle dissolving in the membrane and dissolving in the water that has passed through the membrane.
Reverse osmosis membranes are non-porous. They reject or allow ions and molecules to pass through, by the dissolution-diffusion mechanism. Therefore, reverse osmosis cannot be considered a filtration process.
Membranes are manufactured from synthetic organic polymers, such as: polyamides (PA), polyamides/polyacrylonitrile (PA/PAN), polyethersulfone (PES), polyvinylidene fluoride (PVDF), polysulfone (PSF), polyethylene (PE)…
The membranes retain smaller particles than those retained by the rest of the filter media.
 Figure 1. Comparison of water filtration processes and particle size ranges they retain (Weiner, 2012).
Figure 1. Comparison of water filtration processes and particle size ranges they retain (Weiner, 2012).
What is a solid?
It is a state of aggregation of matter in which the molecules that form it are together, maintain great cohesion and resist changes in shape and volume. Liquids only resist changes in volume. Gases do not resist changes in shape or volume.
What is the smallest particle size of a solid suspended in water?
It is not possible to precisely define the smallest particle size from which it can be considered a solid. The American Water Works Association (AWWA), the Water Environmental Federation (WEF) and the American Public Health Association (APHA) publish a standardized method for determining the total suspended solids content of water in the Standard Methods for the Examination or Water and Waste Water. The method uses a glass fiber filter with a nominal pore size of 1.5 µ. From this perspective, it does not consider suspended solids that are not retained in the filter.
Therefore, the gravimetric method of the Standard Methods for the Examination or Water and Waste Water considers total dissolved solids, not only those actually dissolved, but also those that are not retained in the glass fiber filter.
For reference, the smallest particle detectable by the human eye has a diameter of between 50 and 100 µ (i.e., between 0.0 and 0.10 mm).
The average diameter of human hair is between 70 µ and 120 µ.
What is the range of particle size retained by a water filter?
Through the filtration process, particles ranging from 0.001 microns (in a nanofiltration membrane) to about 2.5 cm (in a basket strainer) can be retained.
Smaller particles can be retained by reverse osmosis. And larger particles are usually retained by metal grids.
What are the most important parameters that define the design of a liquid filter?
Parameter |
What defines the parameter in the filter design? |
Particle size of solids to be retained |
Filter media porosity |
Maximum allowable leakage of solids in the filtered fluid |
Filter media porosity |
Volumetric flow requiring filtration and concentration of solids to be retained |
If these two variables lead to early clogging of the filter media, a cleaning method (backwashing, scraping or other) is necessary. |
Volumetric flow rate requiring filtration |
Filter dimensions |
Chemical properties of the liquid to be treated |
Filter materials of construction (base, housing and filter media) |
Pressure and temperature of the liquid to be treated |
Filter materials of construction (base, housing and filter media) |
What is surface filtration and depth filtration?
Filter media retain particles in two ways:
- Surface filtration occurs when the particles are larger than the pores of the filter media. These are deposited on the outer surface of the medium and form a “cake”.
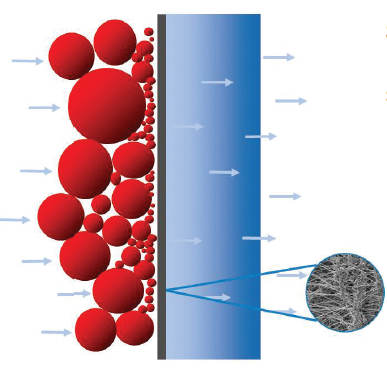
Figure 2. Surface filter. (Donaldson)
2. Depth filtration occurs when the particles are smaller than the pores of the filter media. Retention occurs in the internal structure of the medium.
depth filtration
(
Figure 3
).
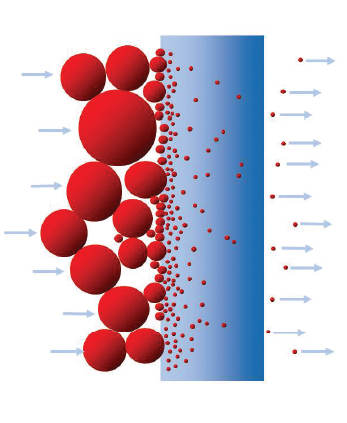
Figure 3. Depth and non-fixed pore filter. (Donaldson)
The surface filters can be cleaned by scraping off the retained cake or by flushing with water.
Depth filters cannot be cleaned efficiently unless the filter media is decompacted.
In depth filtration, granular media are made of multiple layers of media(multimedia). When the fluid passes through the filter, particles larger than the spaces within the filter media are retained, accumulating mainly in the different layers of granular media in the filter.
These are filters formed by fibers or materials compressed to form a matrix that retains the particles in the superficial and internal layer, these can have micronages graduated from higher to lower, to avoid a premature clogging of the cartridge.
For very high flow rates cartridge filters are not the best option, the recommendation is to use deep bed filters or disc filters.
Surface filters are inherently uniform structures that, like a sieve, retain all particles at the same pore size, giving you precise control over their surface, most commonly using bag filters or pleated filters.
References
- Wakeman, Richard J., Solid/liquid separation: principles of industrial filtration, Elsevier, 2005. principles of industrial filtration. Elsevier.
- AWWA, Water Quality &Treatment a Handbook of Community Water Supplies, McGraw-Hill, 1999. Water Quality &Treatment a Handbook of Community Water Supplies. McGraw-Hill.
- Test for total suspended solids. Accessed on the Internet on January 20, 2023 (https://www2.gov.bc.ca/assets/gov/environment/research-monitoring-and-reporting/monitoring/emre/methods/solids_total_suspended_tss_-_pbm.pdf)
- Spanish Association of Desalination and Reuse. Consultado en internet el 20 enero 2023 (https://aedyr.com/diferencias-microfiltracion-ultrafiltracion-nanofiltracion-osmosis-inversa/#:~:text=La%20principal%20diferencia%20entre%20ambos,en%20el%20proceso%20de%20filtraci%C3%B3n).
Preparation and revisions
Prepared: GGC 23 Jan 2023
Reviewed:
Are you interested in learning about a specific filtration separation method?
Keep reading the following articles:
Microfiltration
Microfiltration is a filtration process using a microporous media that retains the suspended solids of a fluid. The pore size of the membrane ranges from 0.1 to 1 micron or microns.
Microfiltration filtration in the market is typically done by cartridge filters, disc filtration and deep bed filters.
Nanofiltration
Nanofiltration is a filtration process using a nanoporous membrane that is used in waters with low total dissolved solids. The purpose is to remove polyvalent ions, in addition to disinfecting it by retaining organic matter.
Ultrafiltration
It is a separation process based on a porous membrane with openings between 0.01 and 0.1 microns.
Ultrafiltration membranes are more closed compared to microfiltration, but more open than nanofiltration and reverse osmosis. The membranes work with low pressure, which results in lower operating costs. In addition, they are very effective as a pretreatment for reverse osmosis, and also have a backwashing system, which gives them a longer life span.
Reverse osmosis.
Reverse osmosis, although it could be called a very fine purification method, is ultimately by filtration at the Angstroms level.
Reverse osmosis (RO) is a process in which the flow rate through a semipermeable membrane is reduced and a buoyancy force in excess of the osmotic pressure is exerted in the opposite direction to the osmosis process (Figure 1). In this way, the substances found in the water are separated on one side of the membrane (concentrate) and on the other side a diluted solution low in dissolved solids is obtained (permeate).
Share:
If you need more information, please contact us.
Some products that may interest you
-
ESNA, NANO and HYDRACoRe Nanofiltration Membrane (NF)
Add to quote -
Hydranautics SWC4 SWC5 and SWC6 Reverse Osmosis Seawater Membranes SWC5 and SWC6
Add to quote -
CPA2, CPA3, CPA5, CPA6 and CPA7 Hydranautics composite polyamide membranes
Add to quote -
RO-4040-FF and RO-390-FF FilmTec DOW DuPont RO-4040-FF and RO-390-FF membranes
Add to quote -
HSRO-390-FF FilmTec DOW 8×40″ Membrane HSRO-390-FF FilmTec DOW 8×40″ Membrane
Add to quote -
Membrane HSRO-4040-FF FilmTec DOW 4×40″
Add to quote -
SanRO HS-4 and SanRO HS-8 4×40″ and 8×40″ Hydranautics Membrane
Add to quote -
Ultrafiltration Membrane HYDRAcap MAX 40, 60 and 80 Hydranautics
Add to quote


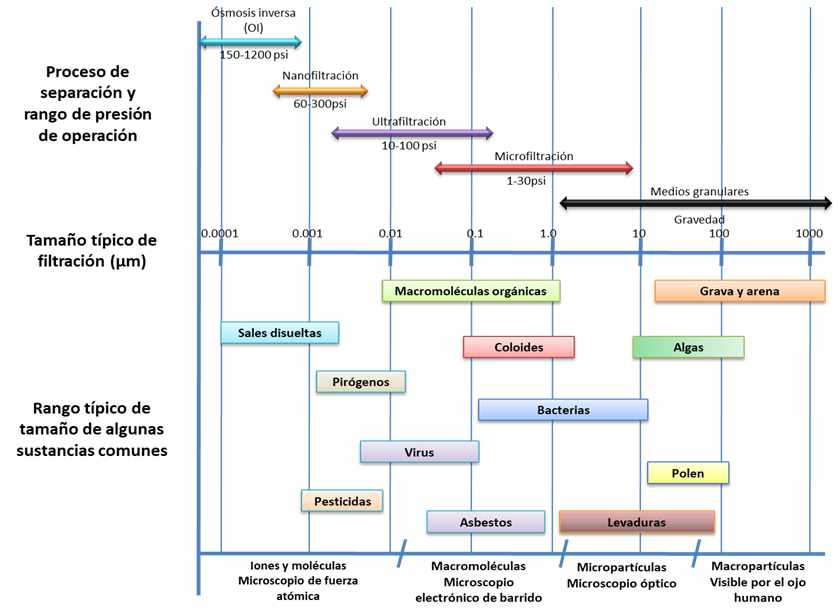 Figure 1. Comparison of water filtration processes and particle size ranges they retain (Weiner, 2012).
Figure 1. Comparison of water filtration processes and particle size ranges they retain (Weiner, 2012).


















