
1. Activated carbon.
Activated carbon or activated charcoal is a porous element that traps compounds, primarily organic, present in a gas or liquid. It does this so effectively that it is the most widely used purifying agent by humans.
On the other hand, organic compounds are derived from the metabolism of living beings, and their basic structure consists of chains of carbon and hydrogen atoms. These include all derivatives from the plant and animal world, including petroleum and the compounds obtained from it.
The property of a solid to adhere a flowing molecule to its walls is called “adsorption”. The solid is called “adsorbent” and the molecule, “adsorbate”.
Keep reading this complete guide to know how activated carbon works, its main applications, and if you read to the end you will find a complete table with references to articles specific to each characteristic of activated carbon. The topics we will address are as follows:
- What is activated carbon?
- What is activated carbon used for?
- Where can activated carbon be obtained?
- How does activated carbon work?
- From which raw materials can activated carbon be obtained?
- Coconut husk charcoal
- Mineral coals.
- What is the adsorption capacity of activated carbon?
- How does activated carbon work in dechlorination?
- Which type of activated carbon is the most suitable for water purification?
- Which type of activated carbon is the most suitable for purifying air and gases?
2. What is activated carbon used for?

(carbon retains pesticides, greases, oils, detergents, disinfection by-products, toxins, color-producing compounds, compounds originating from the decomposition of algae and plants or from animal metabolism…).
For example: in cartridge respirators, air recirculation systems in public spaces, drain vents and water treatment plants, paint application booths, spaces that store or apply organic solvents.
Activated charcoal is considered the “most universal antidote”, and is applied in emergency rooms and hospitals.
- Sugar refining.
The charcoal retains the proteins that give color to the cane juice; the fundamental objective of this process is to prevent the sugar from fermenting and spoiling.
- Discoloration of vegetable oils.
(such as coconut). Corn glucose and other liquids intended for food.
- Discoloration and deodorization of alcoholic beverages.
(such as grape wines and distillates of any origin)
Gold that cannot be separated from minerals by flotation processes is dissolved in sodium cyanide and adsorbed on activated carbon.
3. Where can activated carbon be obtained? Where to buy?
You can buy it in several places in Mexico. To purchase pellet, granular and powdered activated carbon for cosmetic use you can contact an expert here: [email protected]
We also handle activated charcoal for poisoning and indigestion.
How does activated carbon work and what are its benefits?
Activated carbon is an adsorption medium, its function is to adsorb organic molecules in its micro pores. It is activated by thermal or chemical processes to enhance its adsorption capacity (to make pores form).
Image explaining how activated carbon works:
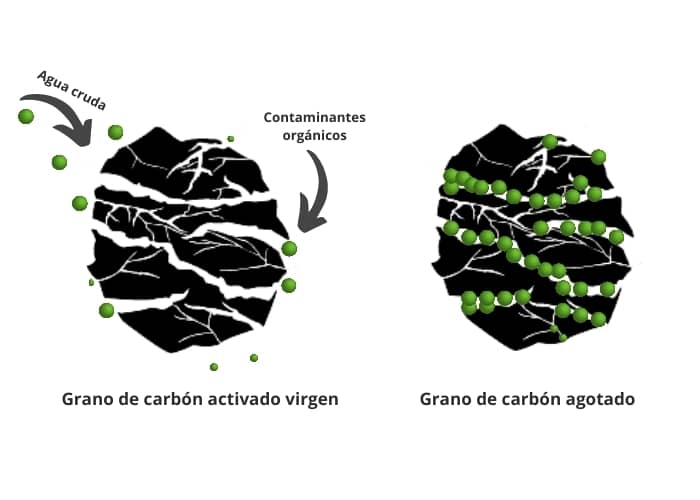
On the other hand, activated carbon is an adsorbent, not an absorbent, as shown in the second image:
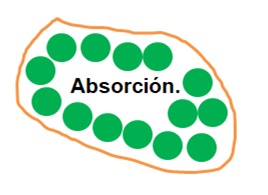
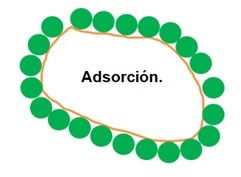
Activated carbon has the ability to adsorb. So, some people put charcoal in the refrigerator to get rid of bad odors. The same happens when you put charcoal in a bucket of water. Eliminates color, taste and odor. Or, in the countryside, people burn and eat tortillas to relieve digestive problems (mild infections, indigestion, bloating, etc.).
Activated carbon involves making it porous to increase its absorbency. One gram of charcoal has a surface area of about 50 square meters. With activation, it reaches 600 to 800 m2, i.e., a 12 to 16-fold increase.
Carbon atom.
The carbon atoms that make up the solid we call “carbon” are held together by covalent bonds. Each atom shares an electron with four other carbon atoms (remember that in an ionic bond, electronegative atoms steal one or more electrons from each other).
Atoms that are not on the surface distribute their four bonds in all directions. But the outer atoms, though bound to four other atoms, are forced to do so in the smaller space, and there are still power imbalances within them. This imbalance is what causes them to trap a liquid molecule surrounding the carbon.
London Force.
The force with which a carbon atom is trapped on the other surface is known as the “London force”, and is one of the seven types of “van der Waals forces”. It is a physical chemical bond, enough to contain the adsorbent material, but not enough to be considered an irreversible chemical bond that forms a new molecular structure. Therefore, adsorption is reversible and the activated carbon can be recycled for reuse.
As we said, the molecules absorbed by carbon tend to be covalent; it changes from ionic, because the latter will try to steal or donate electrons to the carbon atom. The bonds between carbon and hydrogen atoms are covalent, so carbon is a good adsorbent for organic molecules.
Not all organic molecules tend to be covalent. They often contain oxygen, sulfur and other highly electronegative atoms, giving an ionic tendency to the part of the molecule containing them. On the other hand, not all inorganic molecules are ionic; there are also those that tend to be covalent. Such is the case of gold cyanide, which makes activated carbon a fundamental ingredient in the extraction of this precious metal.
5. Raw materials from which activated carbon can be obtained. How is coal formed?
Any carbon particle can be activated (in theory). However, if the carbon is highly ordered (as is the case with diamond or graphite), it is difficult to remove some carbon atoms to generate pores.
One way of classifying coals is based on the degree of “coking” or arrangement of their carbon atoms. The less ordered, the less hard the carbon is and the more easily it can be activated.
6. Coconut shell charcoal and wood charcoal.
The raw materials most commonly used to manufacture activated carbon are: soft woods (such as pine), mineral coals (lignite, bituminous and anthracite) and vegetable shells or pits (coconut shells, olive or peach pits, walnut shells).
Activated carbons made from soft woods form large diameter pores and are particularly suitable for decolorizing liquids.
7. Mineral coals.
These types of carbons are made from mineral carbons, they tend to form a wide range of pores; they tend to be more suitable for applications where the compounds to be retained are of different molecular sizes.
Those based on hard shells or bones form small pores and are used in the treatment of gases or in the purification of water from wells.
What is the physical form of an activated carbon?
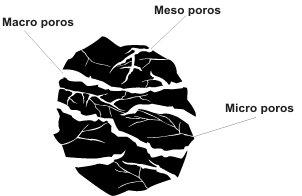
Coal can be produced in the form of powder, granules or cylindrical pellets.
The powder is only applied in the purification of liquids; the carbon is dosed into a tank with agitation and then separated from the liquid by means of a filter suitable for retaining small particles (such as a filter press).
In the case of granular coal, it is produced in different particle size ranges, which are specified based on particle size or mesh number. A 4 mesh, for example, is one that has four holes in each linear inch. They are applied both in the purification of liquids and gases.
Pellets are used in gas treatment, since their cylindrical shape produces a lower pressure drop.
In the case that a granular coal or pellet is desired, if the raw material is not hard enough, it can be reagglomerated with a binding agent that imparts hardness to prevent it from breaking when the fluid passes through.
Activating carbon: How is carbon activated?
Coal can be activated by thermal or chemical processes. Thermal processes to activate coal consist of causing a partial oxidation of the coal, so that pores are formed, but avoiding gasification and the loss of more coal than necessary. This occurs at temperatures between 600 and 1100 °C, and in a controlled atmosphere (achieved by injecting an appropriate amount of water vapor or nitrogen).
Chemical processes start from the raw material before carbonization. The reagents are dehydrating agents (such as phosphoric acid) that break the bonds that bind the cellulose chains together. After this stage, the material is carbonized at a low temperature (about 550 °C) and then washed to remove reagent residues and other by-products.
Furnaces in which a coal is thermally activated or in which a coal is carbonized by pretreatment with a chemical can be rotary or vertical (staged).
What is the adsorption capacity of activated carbon?
The capacity of an activated carbon to retain a given substance is not only given by its surface area, but also by the proportion of pores whose size is adequate, i.e., a suitable little has a diameter of between one and five times the molecule to be adsorbed.
If this condition is met, the capacity can be between 20% and 50% of its own weight.
9. How does activated carbon work in dechlorination?
Dechlorination consists of a complicated mechanism that can follow different reaction paths in which CA can intervene as a reactant or as a catalyst.
Free chlorine can be added to water in the form of chlorine gas, sodium hypochlorite solution, or calcium hypochlorite tablets (granules). In either case, the chlorine is dissolved in the form of hypochlorous acid (HOCl), a weak acid that tends to partially dissociate.
The distribution between hypochlorous acid and hypochlorite ion depends on the pH and concentration of these species. Both molecular forms are defined as free chlorine.
Both are strong oxidizers which, when added to water, react almost immediately with organic and inorganic impurities and exert a biocidal effect on microorganisms.
The chlorine that reacts and intervenes in this disinfection stage is no longer free and remains combined and is no longer free. Once this stage is completed, it is necessary to eliminate the residual free chlorine by means of granular activated carbon.
When carbon is exposed to free chlorine, reactions take place in which HOCl or OCl- is reduced to chloride ion. This reduction is the result of different possible reaction paths.
In two of the most common, CAG acts according to the following reactions:
Where C* represents activated carbon. C*O and C*O2 are surface oxides, which gradually occupy spaces that, being blocked, no longer participate in the reaction. Some of these oxides are released into solution as CO and CO2. This again leaves available spaces that therefore increase the capacity of the CAG for this reaction.
As for Cl-, it also accumulates on the coal surface during the first moments of operation. As HOCl or OCl- continues to reach the carbon surface, the reaction slows down a bit, and then Cl- begins to be released. This slowdown is due to the poisoning of the coal with surface oxides. This poisoning continues gradually, while the adsorption and dechlorination capacity of the AC decreases.
In the above reactions it can intervene in place of HOCl, with the difference that no H+ is produced. It can be observed that the AC reacts and therefore disappears. If there were no accumulation of surface oxides, the reaction would continue until the complete disappearance of the carbon.
Which type of charcoal is the most suitable for bleaching?
Colors that manifest themselves in liquids are usually large molecules. Therefore, they are adsorbed in large pores, which means that the most suitable carbons to retain them are those with higher macroporosity.
Wood charcoals, especially those of not very hard woods (such as pine) that are chemically activated, are the most macroporous and, therefore, are the most suitable for decolorizing.
The problem with these carbons is that they are not very hard and not very resistant to abrasion, which makes it necessary to apply them in powder form. When the decolorizing coal is required to be granular, the best alternative is usually a lignite coal. It is the mineral coal with the highest macroporosity.
10. Which type is the most suitable for water purification?
The most common contaminants in well water are usually of low molecular weight and, for these cases, the most suitable carbon is one with high microporosity.
The coals that best meet this condition are, firstly, coconut shell coals and, secondly, bituminous minerals.
Why does the pH of the water vary when virgin carbon is installed?
When a carbon is chemically activated, it is impractical and unnecessary for the manufacturer to remove all the chemical used from the final product. Therefore, if the chemical was an acid, it will lower the pH of the first few liters of water that come in contact with the coal. The opposite will occur if the chemical used was an alkali.
In the case of a thermally activated carbon (without the presence of chemicals other than water vapor and combustion gases), it increases the pH of the first few liters of water treated with it. This is because all vegetables have significant amounts of sodium, potassium, calcium and other cations that, in the carbonization process, remain in the charcoal in the form of oxides. These oxides become hydroxides when they come into contact with water, dissolve in water and increase its pH.
When the pH of the first few liters of water that come into contact with a carbon does not vary, it may be a pH-adjusted carbon or an ultrapure (soluble-free) carbon.
11. Which type of activated carbon is the most suitable for purifying air and gases?
All gaseous contaminants have molecular diameters less than 2 nm. This means that they adsorb better in micropores. Coconut shell coals have the highest microporosity and are therefore the most widely used in air and gas purification.
There are activated carbons of modified structure, special activated carbon, which are used when a standard activated carbon cannot retain other non-organic compounds.
At Carbotecnia we are manufacturers and specialists in activated carbon. You can consult the different types we handle
here.
More articles:
Activated charcoal tablets or capsules and other uses
In recent times different products have become famous that make use of activated charcoal for different types of purposes, in some cases activated charcoal is used for supposedly slimming and even for facial care that could probably be addressed with more appropriate solutions. In this article we are going to review what activated charcoal pills can do for our health.
One of the products that became more famous thanks to social networks were activated charcoal tablets . Many young people mention that they take them to get rid of the famous hangover, others argue that their use has helped them to lose weight, but do these revolutionary activated charcoal pills really work?
Also: Is it advisable to use activated charcoal as a medicine?
Continue reading this article to learn more:
https://www.carbotecnia.info/pastillas-de-carbon-activado/
Need a quote, write to us.
Some products that may interest you
-
AA-3 Activated carbon to reduce color and flavor in tequila and other distilled spirits
Add to quote -
Micro K Coconut shell activated carbon
Add to quote -
Micro 4 LF Coconut shell activated carbon free of fines
Add to quote -
Megapol C – Wood powder activated carbon
Add to quote -
Megapol E – Wood powdered activated charcoal
Add to quote -
Micropol 4 200 – Coconut shell powdered activated carbon
Add to quote -
Mega – Wood granular activated carbon
Add to quote -
Gama L – Lignite coal mineral activated carbon
Add to quote


























