What is Electrodeionization (EDI)?
Electrodeionization or electrodeionization is a new technology, which is a combination of electrodialysis and ion exchange. Its function is to remove ions from water. The technology consists of a combination of ion exchange resins, both cationic and anionic, ion-selective membranes, an anode, a cathode and the cyclic application of an electric potential.
In combination with reverse osmosis (RO), EDI as a final stage allows obtaining deionized water with a much lower concentration of salts than that produced by RO. This system replaces the mixed resins that have been used up to now. This means replacing the chemicals required to regenerate resins with electrical current used by EDI.
How does electrodeionization work?
Unlike deionization, here the water is fed into chambers containing the resin, which are bounded by selective membranes, anionic at one end and cationic at the other. At the same time that water is fed into these chambers, an external current source supplies a continuous electric field through electrodes placed at the ends.
The resins retain the cations and anions present, and simultaneously release H+ and OH–. The resins do not act as an ion store, but as a conductor of ions. As part of the cycle, the resins regenerate, and the released cations and anions pass through the membranes and move towards the anode or cathode, as appropriate; on their way, a rejection flux carries them away.
Ion-selective membranes are made of inert polymers and have the same mechanism of operation as ion exchange resins. They only allow the passage of anions or cations, depending on the case, and are impermeable to water.
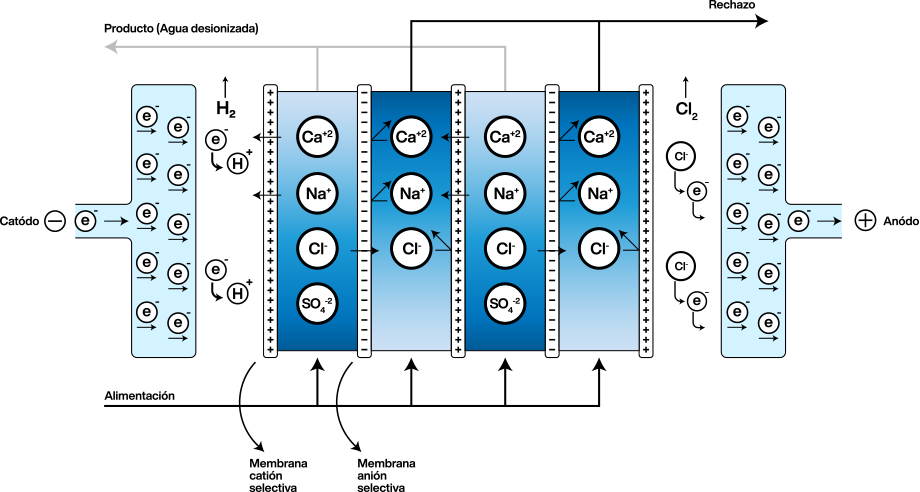
Typical configurations
2. Pretreatment, followed by two consecutive reverse osmosis (RO) steps, and as a final stage, an EDI module. Rejection of dissolved ions in the product water: up to 99.99%.
A possible modification in the two previous ones, consists of incorporating a second EDI step that increases the percentage of dissolved ions rejection. Another is the recirculation of the reject flow from the EDI (or both EDIs) to the reverse osmosis (RO) feed.
The most appropriate configuration will depend on the quality of the feed water to the system, the quality of the product water required and the cost benefit of each.
EDI applications
Currently, EDI is the most competitive alternative for any application requiring water with a low salt concentration (Resistivity of 1-18 MΩ/cm).
Electrodeionization applications:
- Pharmaceutical Industry
- Laboratories
- Electronics and semiconductor industry
- Any industry requiring a product to be washed to a very low salt content (metal parts, jewelry, some injected plastic products, etc.).
- Biotechnology industry
- Chemicals Industries
- Cosmetics industry
- Boiler and heat exchanger feed water
- Etc.
Advantages:
- Low energy consumption.
- It can operate continuously.
- It does not require the use of chemicals for regenerations.
- Compact systems that do not require much space.
- The best cost-benefit ratio, compared to other alternative technologies
- Stability of product water quality.
- Environmentally friendly, as it does not produce effluents with polluting chemicals.
- In combination with reverse osmosis, it removes up to 99.9% of dissolved ions.
Disadvantages of electrodeionization:
- Requires effective pretreatment: conductivity < 40 µS/cm and TOC < 0.5 mg/L.
Bibliographic references:
Jane Kucera, Desalination Water from water, 1st Ed, Scrivener Publishing, U.S.A., 2014.
Philip Murray, Bill Cobban and Kathleen Faller, Electrodialysis and Electrodialysis riversal1st Ed, American Water Works Assosiation, Denver, 1995.
EDI modules available:
Water demineralization modules
Add to quote: Electrodesiotizer (EDI) for ultrapure water
$1.00Add to quote








