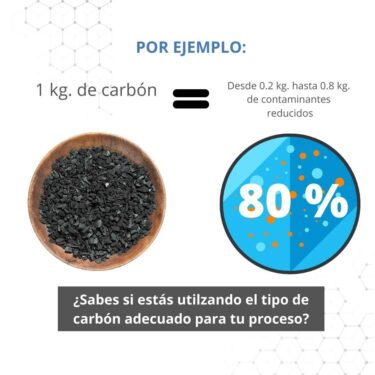
Description
Granular activated carbon for acid gases
Raw material: Coconut shell
Particle size range (mesh): 4×10, 8×14, 14×30, or 4 mm pellet.
Packaging: 25 kg sacks, 100 kg bags, 100 kg bags
Some applications to eliminate acid gases:
- Cartridge respirators (face masks)
- Drainage odors
- Desulfurization of biogas and natural gas.
- Air treatment in spaces where corrosion caused by acid gases must be avoided.
- Control in electronic components production areas
Vapacid type activated carbon is manufactured from coconut shell and impregnated with metal oxides that give it a high capacity for the removal of gases and acid vapors present in air. These acids can be organic such as acetic acid or mercaptans or inorganic such as sulfur dioxide and sulfuric, nitric, hydrochloric and phosphoric acids.
In the case of hydrogen sulfide removal, Vapacid catalyzes the H2S – oxygen reaction:
2 H2S + O2 = S8 + 2 H2O
Unlike other impregnated carbons, Vapacid minimizes sulfuric acid formation by inhibiting sulfuric acid-producing side reactions.
VAPACID adsorbs more than twice as many mercaptans as a non-impregnated carbon, since it oxidizes them to disulfides (2 RSH R2S2), which are less odorous on the one hand, and more easily adsorbed on the other.
Among the main applications of this product are: odor control in drain vents (called louvers) and in wastewater treatment plants; corrosion control and protection of electronic components in aggressive industrial environments; environmental control in museums or in specialty manufacturing areas, such as computer chips or pharmaceutical products; environmental control in processes using mineral acids.
Retains:
– All types of vapors and acid gases.
– All types of organic vapors (although with lower capacity than non-impregnated carbon).
Compound with which organoleptic tests are suggested:
– Hydrogen sulfide (in gas phase it is called hydrogen sulfide:H2S: it is the typical rotten egg smell. It is produced by reacting ferrous sulfide with sulfuric acid).
Like all inorganic acids, it is a toxic compound, so it must be handled with care and in low concentrations.
Among acid vapors and gases, those mainly encountered in industry, commerce and society are:
Acetic acid
Acrylic acid
Adipic acid
Benzoic acid
Hydrobromic acid (HBr)
Butyric acid
Caprylic acid
Caproic acid
Carbolic acid (carbolic acid, phenol)
Cyclohexanecarboxylic acid
Hydrochloric acid (HCl) (also called muriatic acid)
Stearic acid
Ethyldimethylacetic acid
Phenylacetic acid
Hydrofluoric acid (HF)
Formic acid
Phosphoric acid (H3PO4)
Isobutyric acid
Lactic acid
Lauric acid
Linoleic acid
Linolenic acid
Myristic acid
Nitric acid (HNO3)
Oleic acid
Palmitic acid
Perchloric acid
Propionic acid
Hydrogen sulfide (H2S) (typical rotten egg smell)
Sulfuric acid (H2SO4)
m-toluic acid
O-toluic acid
P-toluic acid
Uric acid
Valerianic acid
Valeric acid
Hydroiodic acid (HI)
Hydrogen bromide (the correct name for hydrobromic acid in the gas phase)
Hydrogen chloride (the correct name for hydrochloric acid in the gas phase)
Sulfur dioxide (SO2)
Chlorine dioxide (ClO2)
Phenol (carbolic acid, carbolic acid, carbolic acid)
Hydrogen sulfide (the correct name for hydrogen sulfide in the gas phase)
Hydrogen iodide (the correct name for hydrogen iodide in the gas phase)