Procedure for disinfecting granular activated carbon beds using chlorine dioxide.

Granular activated carbon (CAG) has two functions in a water treatment train: (a) Adsorb the organic compounds that are present in all natural water bodies (well, lake, river); and (b) Removing residual free chlorine that was applied as a biocide in a previous step. Learn in this article how to perform activated carbon disinfection.
Bacteria do not create resistance to free chlorine. Therefore, the chlorinated water that reaches the CAG tank does not contain active bacteria. However, sooner or later, the water that comes out of the equipment, already without free chlorine and without organic pollutants, shows bacterial activity. The reason for this is explained below and the method for activated carbon disinfection using chlorine dioxide is presented.
Why should coal be sanitized?
Due to the fact that CAG adsorbs organic compounds, it becomes a suitable substrate for bacterial development. The rough and cracked surface of the charcoal makes it easy for bacteria to stick. When chlorinated water comes into contact with the top of the bed, the charcoal reacts with the free chlorine and its disinfecting effect disappears. Since this reaction is rapid, no free chlorine reaches the middle and lower part of the carbon bed.
By stopping the water treatment process, equipment is often depressurized. If the water that feeds the AGC tank comes from a lower level and the check valves in the pipe do not close properly, a vacuum and a backflow are generated. This can cause the entry of microorganisms that come from the water outlet points, the sampling valves or any leak. If these microorganisms reach the coal, they fixate in its cracks and feed on the adsorbed organic matter.
When starting the process again, the free chlorine does not affect them. They multiply form a biomass that the water ends up dragging. This is how the time comes when the equipment effluent carries bacteria. If this water goes through a UV or ozone disinfection stage, subsequent bacteriological analyzes will not show bacterial activity, but the time will come when the water will smell like a drain.
When is it advisable to sanitize the activated carbon bed?
This is why, in a water treatment train intended for human consumption, bacteriological analysis of the effluent from the CAG equipment must be included in the quality control protocol, right at its outlet. When these analyzes do not comply with the potability standards, it is necessary to disinfect the coal bed.
Disinfection of charcoal should be done as efficiently as possible to delay reinfection as long as possible. The absence of leaks, the pig tails at the sampling points, the remoteness of the treated water intakes from the equipment with CAG, well-closed valves and no leaks, as well as microbiological barriers (such as UV) decrease the frequency with the that will need to be disinfected.
In the case of home water purifiers, in which the application of any of a disinfection method is not practical, the CAG impregnated with silver, which is bacteriostatic, is used. This is not recommended in industrial processes because it is expensive and silver is lost long before the capacity of the CAG is exhausted. Remember that the coal in a home purifier operates for a few minutes a day, while that of an industrial process operates for hours. Therefore, in the case of industrial processes, it is indicated to disinfect properly.
With what chemical can I disinfect activated carbon?
Effective disinfection of a CAG cannot be achieved by oxidants such as chlorine, ozone, or hydrogen peroxide. The CAG destroys them quickly and they do not reach all points of the bed. Therefore, part of the microorganisms remain active.
The only inorganic biocide suitable for disinfecting CAG beds is chlorine dioxide.
Method to disinfect a CAG bed using chlorine dioxide.
The procedure to disinfect with this compound is as follows:
1.- Calculate the volume of ClO solution2 required. This should completely flood the CAG bed. CAG beds typically have a porosity of around 40%. That is, the 40% of the volume of the bed is empty space (formed by the space between the carbon particles and by the space within the pores). The 60% of the remaining space is taken up by the solid. Gravel beds or support gravel have a porosity of about 30%.
Calculation example:
The CAG contained in a 48” diameter, 72” high fiberglass tank is required to be disinfected. The coal is placed on a bed of support gravel. The volume of the CAG is 40 ft3 and the volume of the gravel is 6 ft3. It is required to completely flood the bed with CAG, and leave a 40 cm supernatant. Calculate the volume of chlorine dioxide solution to prepare.
Calculation of the volume of supernatant solution:
Inner diameter of the tank (we will assume that it is equal to the nominal diameter) = 48”.
Tank inner diameter = 48” = 4 ft
Tank cross-sectional area = 3.1416 x 42/ 4 = 12.57 ft2
Height of supernatant solution = 40 cm = 1.31 ft
Volume of supernatant solution = 12.57 ft2 x 1.31 ft = 16.50 ft3
Calculation of the total volume of the solution to be prepared:
Empty space in CAG bed: 40 ft3 x 0.4 = 16.00 ft3
Gravel Bed Empty Space: 6 ft3 x 0.3 = 1.80 ft3
Volume of supernatant solution: 16.50 ft3
—————————————————————————————————————————————————————————————————-
Total volume of solution to prepare: 34.30 ft3
971 liters
2.- In a separate tank, prepare the chlorine dioxide solution at a concentration of 30 mg / L. Shake well so that the solution is homogeneous.
Chlorine dioxide is usually obtained locally, as it evaporates at 11 ° C and decomposes in sunlight. It is typically obtained from a chemical reaction between a solution of sodium chlorite at 25% (Approx.) And a solution of hydrochloric acid at 4%. These two solutions are reacted in a 1/1 ratio (the same volume of each). One milliliter of each solution produces 45 mg of chlorine dioxide.
For the case of the example in part 1, 971 liters of chlorine dioxide solution at a concentration of 30 mg / L are required. Therefore:
Chlorine dioxide mass required = 30 mg / L x 971 L = 29 130 mg ClO2
Therefore,
The Required volume of sodium chlorite solution at 25% =
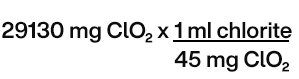
For required volume of sodium chlorite solution at 25% = 647 mL
Required volume of hydrochloric acid solution at 4% = 647 mL
3.- Backwash the CAG tank in order to unpack the bed. It is important that the expansion of the same is achieved, so that the chlorine dioxide solution can come into contact with all the carbon granules.
4.- Empty the CAG tank.
5.- Make the chlorine dioxide solution flow into the tank to be disinfected.
6.- Let stand for at least an hour.
7.- Backwash and rinse.

Comparte:
Necesitas más información, escríbenos.
Algunos productos que te pueden interesar
-
AA-3 Activated carbon to reduce color and flavor in tequila and other distilled spirits
Add to quote -
Micro K Coconut shell activated carbon
Add to quote -
Micro 4 LF Coconut shell activated carbon free of fines
Add to quote -
Megapol C – Wood powder activated carbon
Add to quote -
Megapol E – Wood powdered activated charcoal
Add to quote -
Micropol 4 200 – Coconut shell powdered activated carbon
Add to quote -
Mega – Wood granular activated carbon
Add to quote -
Gama L – Lignite coal mineral activated carbon
Add to quote






















